Closed-loop Seizure Control for Epilepsy
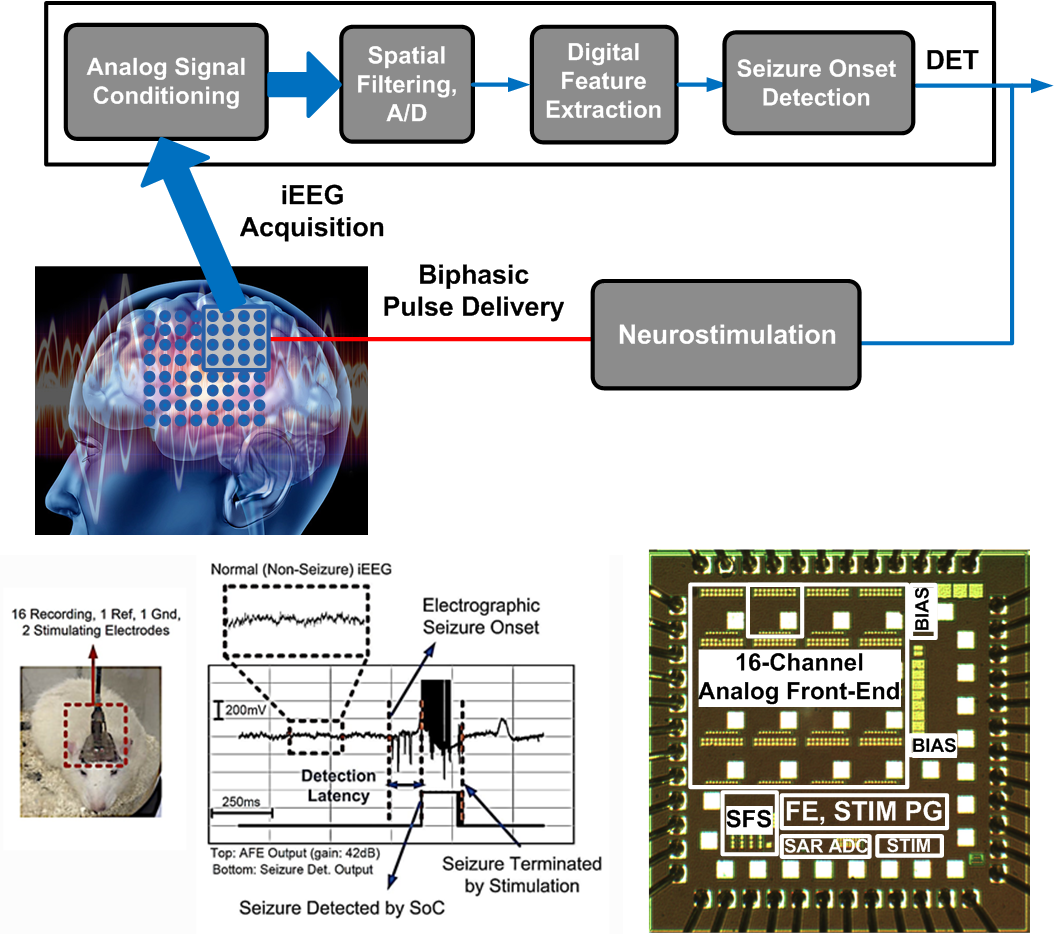
We develop new ways of personalized monitoring and treatment for early detection and prevention of disabling neurological conditions using smart implantable and wearable technologies. In a closed-loop neural interface, such as a seizure control system, the neural signals recorded by an array of electrodes are amplified, filtered and digitized. A feature extraction processor is subsequently activated to extract the disease-associated biomarkers. Upon abnormality detection, a programmable neural stimulator is triggered to suppress the symptoms of disease (e.g. a seizure or migraine attack, Parkinson’s tremor, memory dysfunction, etc.) through periodic charge delivery to the tissue. For example, our latest seizure control system-on-chip (SoC) designed for patients with medication-resistant epilepsy remarkably improves the state-of-the-art, in terms of both precision and hardware usage.
Machine Learning Hardware for Diagnostic Applications
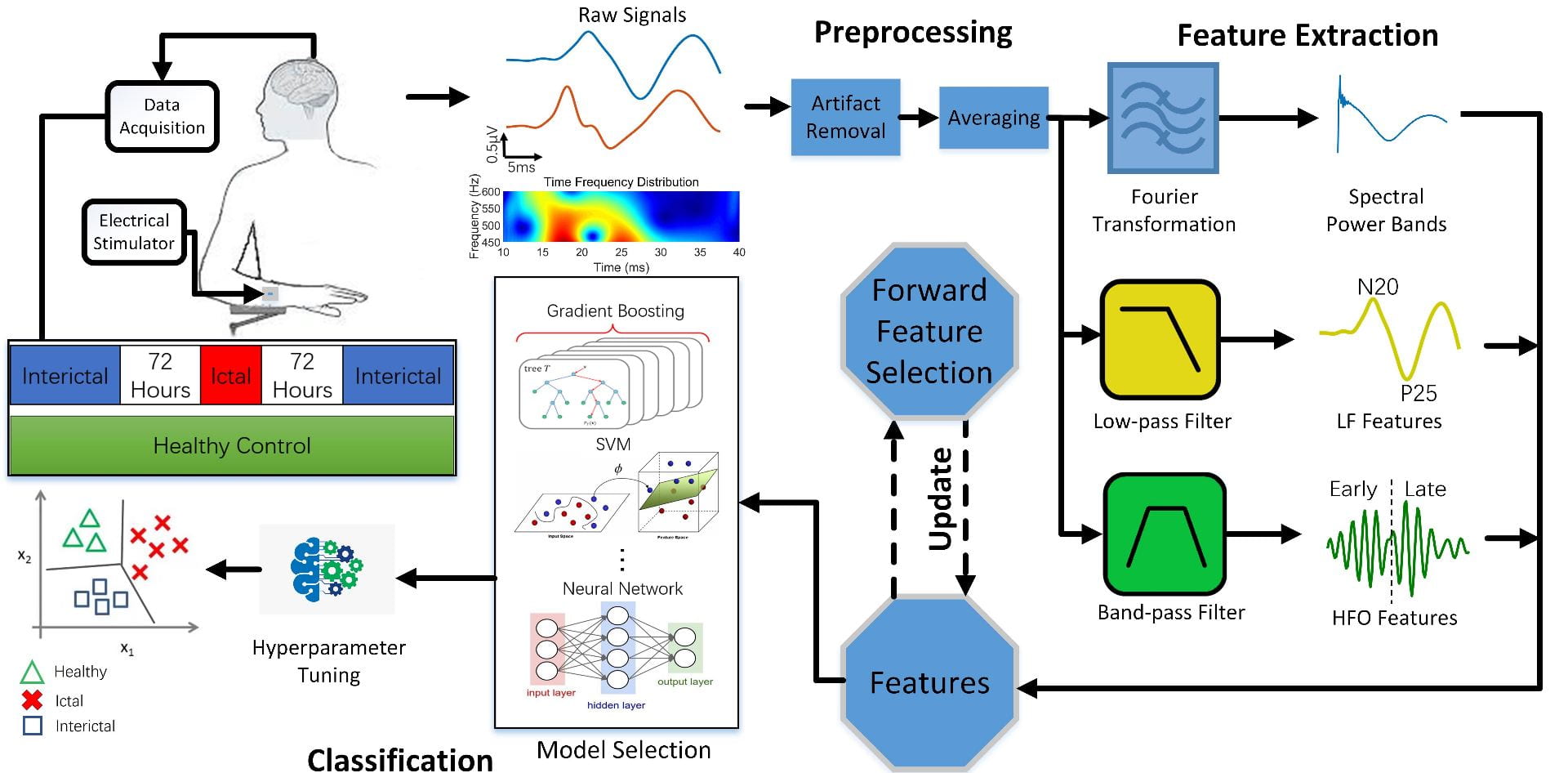
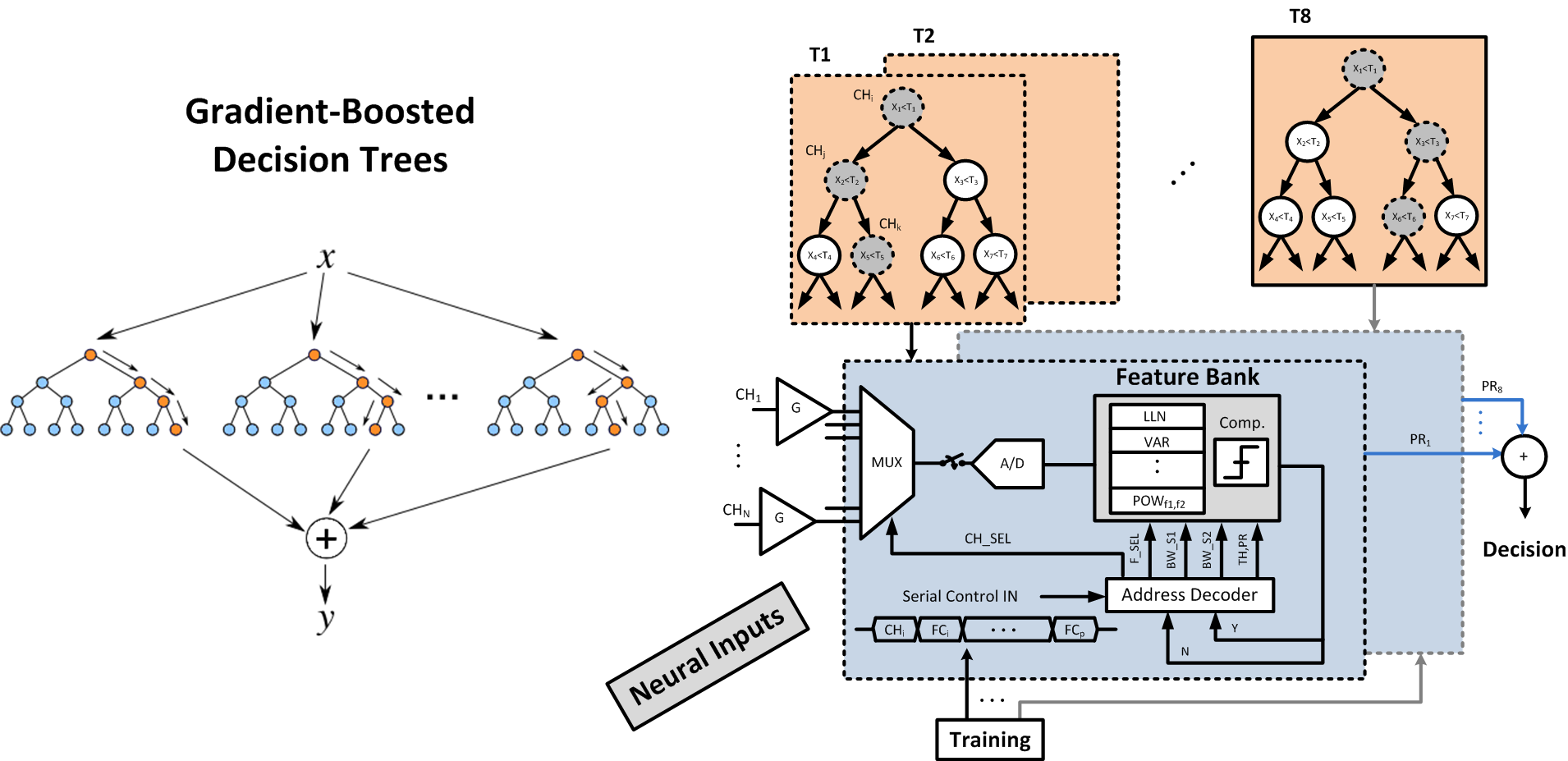
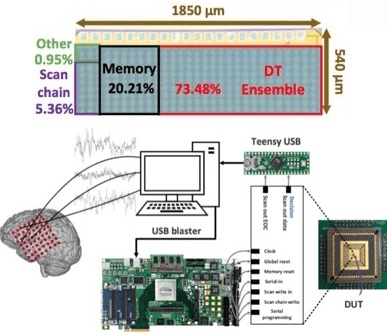
We explore a class of simple, yet sufficiently accurate learning algorithms that allow full integration of processing circuitry with the sensor array in various biomedical applications. Our preliminary results on seizure onset detection suggest that these gradient-boosted ensembles of axis-parallel decision trees are excellent candidates for on-chip integration, with a simple hardware proposed here. Combined with the implemented feature extraction model, these classifiers quickly become competitive with more complex learning models, using only a small number of low-depth shallow trees. We have implemented this architecture in a 65nm TSMC process for epileptic seizure detection. The fabricated SoC was successfully tested on pre-recorded iEEG data (32-channel) from 20 patients, achieving a 41.2nJ/class energy efficiency in a total area of 1mm2, improving the SoA neural classifier by 30× in energy efficiency and 28× in area usage per channel.
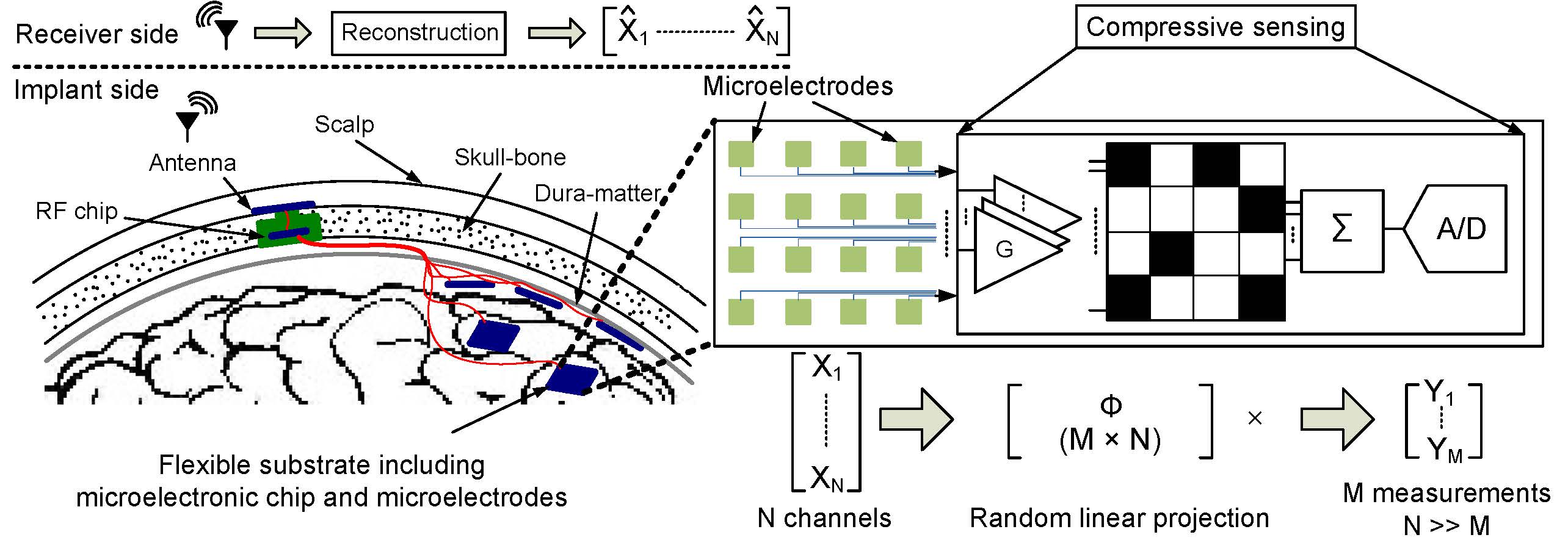
Compressive Sensing for High-Density iEEG Recording
The application of compressive sensing (CS) theory has shown promising results for sub-Nyquist sampling of biological signals. However, the occupied die area of a CS system can be significantly larger than a typical acquisition system without compression. To solve this issue, we have proposed an efficient approach to compressive acquisition of cortical signals in wireless neural prostheses. We implement a multichannel CS scheme which exploits the spatial sparsity of the signals recorded from the electrodes of the sensor array. The proposed approach preserves the power efficiency while significantly reducing the area overhead. This design improves the power dissipation of the wireless transmission, and hence boosts the number of channels that can be integrated on an implantable chip.
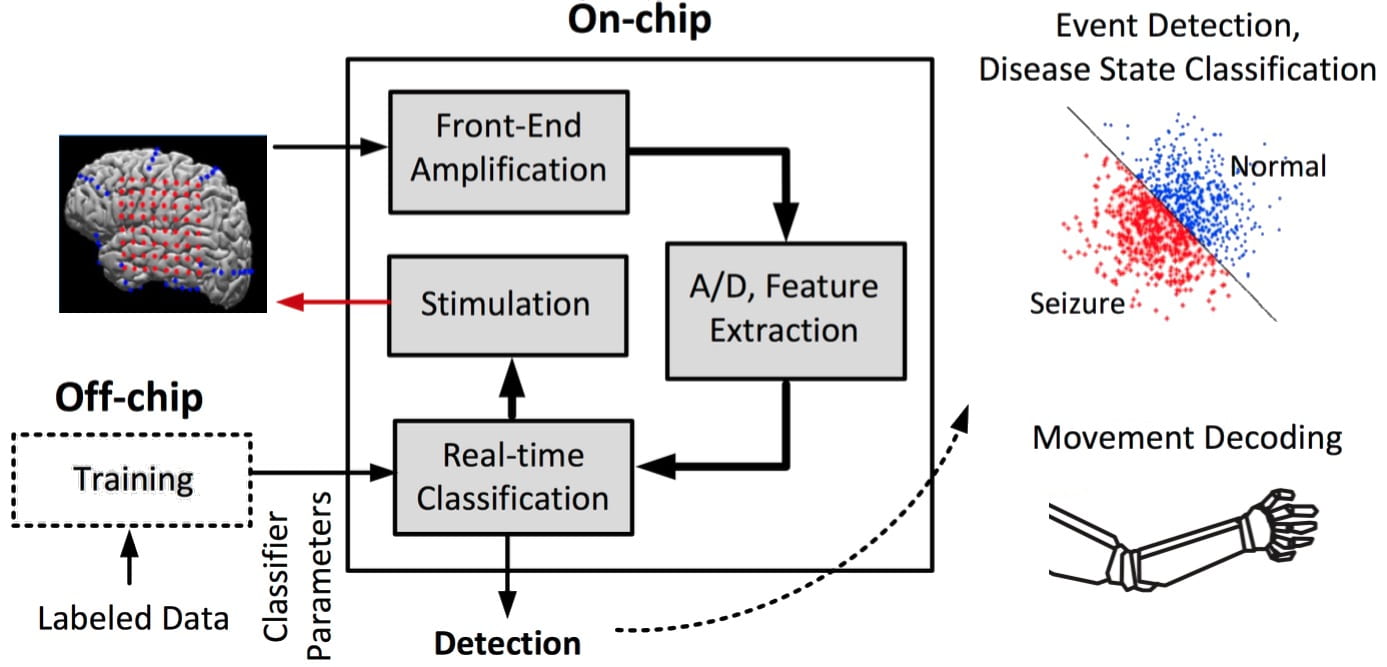
Closed-loop Neural Implants and Brain-Machine Interfaces
Millions worldwide suffer from motor-related injury or chronic neurological diseases that remain largely undertreated. As an emerging therapeutic alternative, neural devices and prostheses are increasingly being explored to detect neurological abnormalities in real-time, and trigger a therapy such as neurostimulation to restore normal function. Moreover, brain-machine interfaces (BMIs) hold the promise to ultimately restore motor function through cortical or spinal cord stimulation. Clinical studies currently require multiple computers and recording systems to function, resulting in bulky hardware that is not convenient for daily use. A bottleneck for local processing is the lack of low-power and area-efficient classifiers that can accurately predict the symptoms of disease or decode a motor task in real-time. We propose to employ state-of-the-art ML principles and efficient hardware architectures to enable real-time neural data processing on the implant. This project is funded by a 2018 Google Faculty Research Award in Machine Learning.
Migraine State Classification
Migraine is the most common neurological disorder, characterized by recurrent headache attacks. Despite its high prevalence, migraine diagnosis is still mainly based on clinical interviews and patient diaries. Automatic detection of migraine state using electrophysiological recordings may play a key role in migraine diagnosis and early treatment. Migraineurs are characterized by a deficit of habituation in cortical information processing, causing abnormal changes of somatosensory evoked potentials (SSEPs). We recently used an ML approach to utilize SSEP biomarkers for migraine state prediction in a noninvasive setting. Our analysis shows that high-frequency oscillations (HFOs) embedded in SSEP are important biomarkers for migraine state prediction. Using a set of relevant features, we successfully separated migraine patients during and between attacks from healthy controls with over 80% accuracy. This is a collaborative project with the Neurophysiology Department at IRCCS in Rome, Italy.
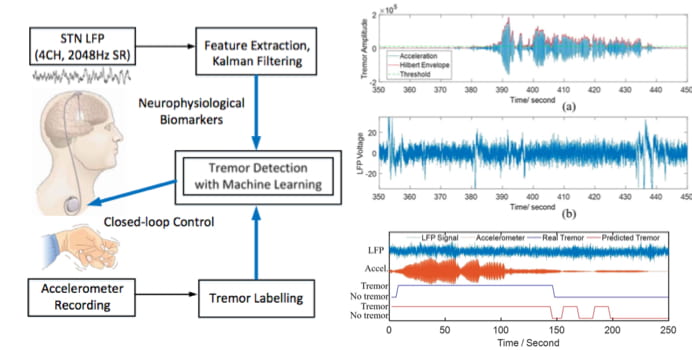
Parkinsonian Tremor Detection for Adaptive DBS
Parkinson patients treated by the conventional DBS may suffer from side effects such as speech impairment and psychiatric symptoms. Moreover, open-loop stimulation increases the energy consumption of the DBS device, which may require a surgical replacement procedure. Alternatively, the concept of adaptive DBS (aDBS) holds the promise to alleviate side effects and improve the efficacy of conventional open-loop DBS. However, current aDBS techniques are primarily based on single-feature thresholding, precluding an optimized delivery of stimulation and precise control of motor symptoms. We used multiple features of local field potentials (LFPs) recorded from STN, and combined it with machine learning and Kalman filtering to improve the accuracy of detecting resting tremor in Parkinson’s. We found that by optimal selection of only five features using a forward feature selection method, the system can achieve an average F1 score of up to 91.8%. This is a collaborative project with the Clinical Neuroscience Department at the University of Oxford.